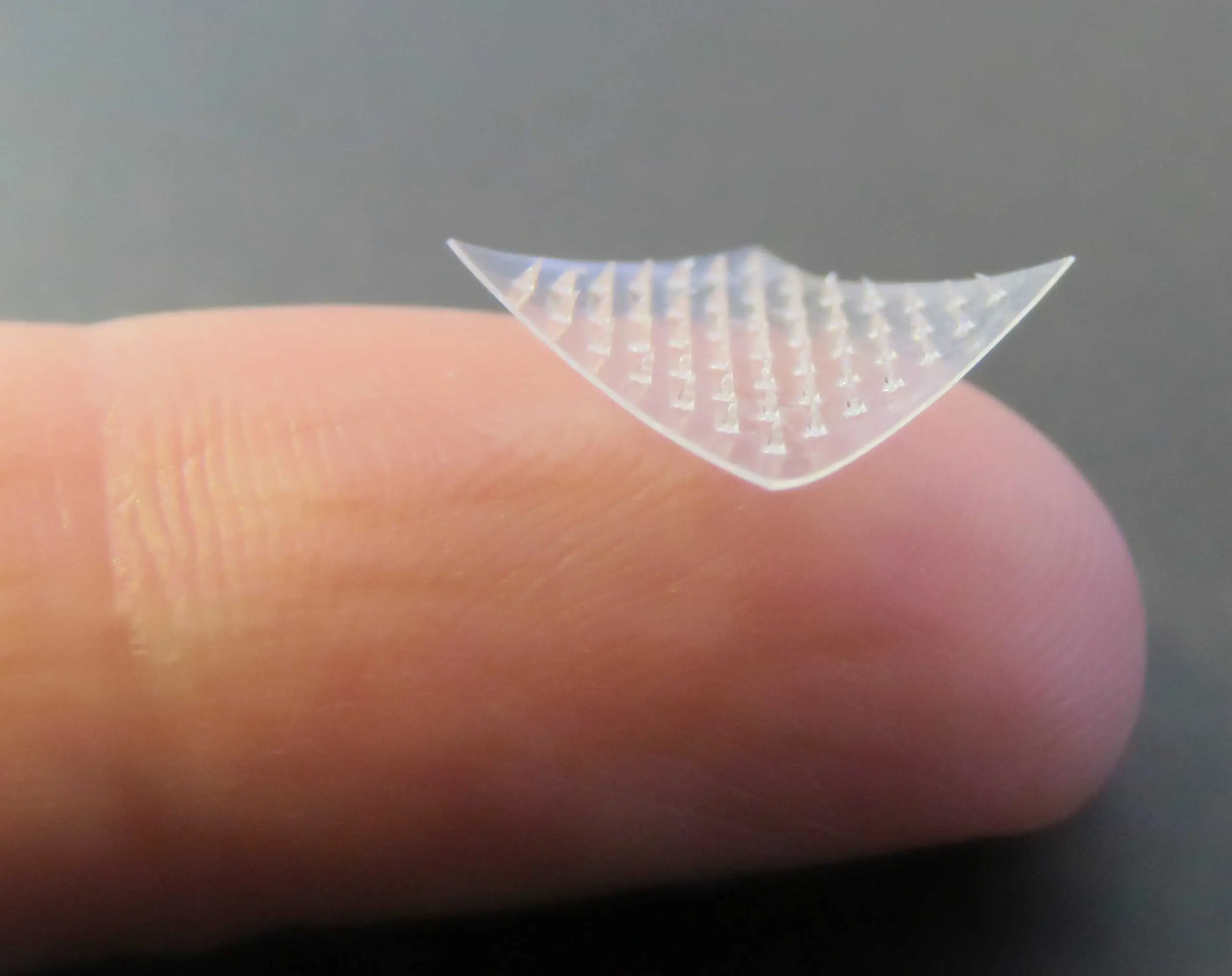
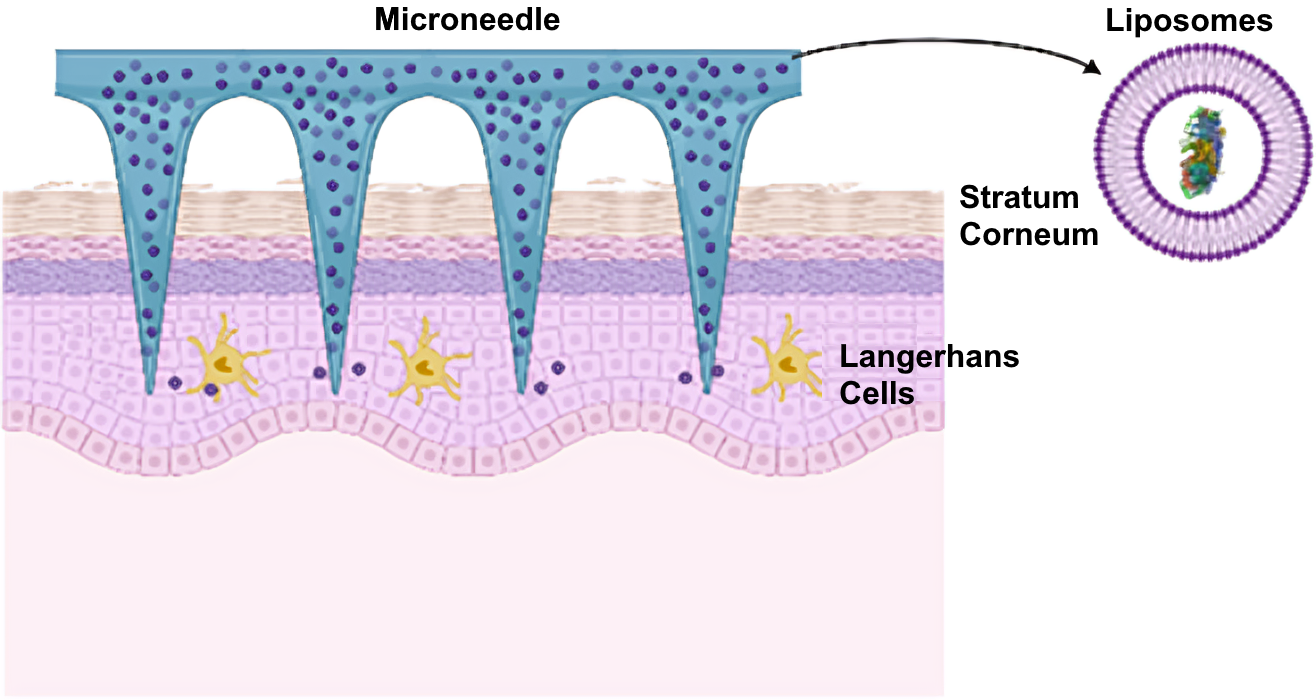
Introducing breakthrough nanotechnology microneedle patch, designed to desensitise food allergies without the risk of oral exposure.
Food allergies like peanuts, eggs, and milk affect millions worldwide—often with life-threatening consequences. At Zallergen, we’ve pioneered a new approach: Epicutaneous Immunotherapy using a patented dissolving microneedle patch that delivers allergens through the skin, avoiding the risks associated with Oral Immunotherapy (OIT).
Developed after a decade of research at the University of New South Wales (UNSW) by Associate Professor Alice Lee. Our technology offers a smarter, safer alternative to oral immunotherapy. The patch’s microneedles painlessly breach the skin barrier, while our proprietary nanotechnology ensures ultra-precise dosing and controlled allergen delivery, minimising risk while maximising efficacy *.
Note: * clinical trials have not been completed.
Novel nanocarriers utilising peanut allergen-loaded liposomes are promising nanomaterials showing great potential as a vehicle for skin delivery in the context of EPIT.
Epicutaneous Immunotherapy or patch-based immunotherapy, has shown consistent evidence of efficacy, safety, and high treatment adherence in children with peanut allergy.
Increasing reactivity threshold in peanut-allergic children aged 4 to 11 years ... may also reduce the severity of allergic reactions to accidental peanut ingestion.
Peanut-coated microneedle therapy was found to be safe and demonstrated superior desensitization outcomes compared to peanut EPIT administered on a comparable schedule.
Zallergen was born from collaboration at the UNSW RNA Institute, a nationally recognised hub within the NSW Government’s $96 million RNA ecosystem. At its core is the RNA Accelerator, Australia’s first ISO 9001‑certified facility for pilot-scale manufacture, design, and development of RNA-based therapeutics and pre-clinical products. The Accelerator supports industry and academia with technology in drug delivery, RNA interactions, and manufacturing. We are one of the earliest companies in this network with microneedle capabilities, strategically positioned to leverage the infrastructure, and expertise to translate the next-generation vaccines and biologics.
If you have a food allergy or know someone who does, sign up now to register your interest and stay informed.
The next step involves completing a short profile questionnaire to help us assess and screen your eligibility.
If you meet the qualifying criteria after assessment, you'll be assigned to the most convenient clinical trial centre.
Interested in learning more about our product, or participating in a clinical trial:
Copyright ⓒ 2026 Zallergen